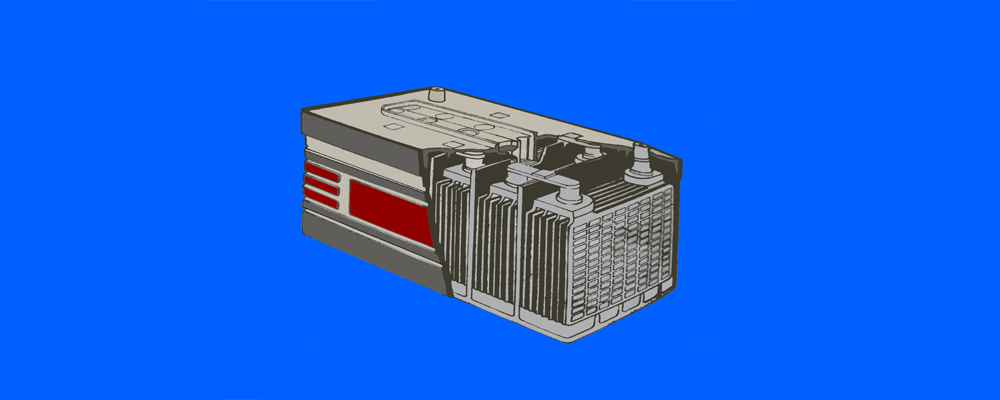
Automotive Battery: What Is A Battery?
The vehicle’s electrical system comprises many different modules. However, the automotive battery is the heart of the vehicle’s electrical system. It supplies the initial current to the starting and ignition systems. When the generator/alternator is not charging (engine stopped), the battery always provides the current to the other electrical devices. Besides, it acts as a secondary source of electric current when the vehicle is not operating. Or the generator/alternator speed is not sufficient to meet the requirements.
The Capacity Of The Battery:
Over some time, the battery got updates and upgrades. Thus, it changed its appearance and capacity. However, the primary function of the battery mainly remained unchanged. However, the battery now needs to be of a substantially higher capacity. It is because the number of components operating on electric power also increased.
Actually, the capacity of a conventional battery depends upon the amount of chemicals it contains. Thus, it also limits the amount of current it can supply. Lead-acid, Nickel-alkaline, and Zinc-air are vehicles’ most popular battery types.
The Function Of An Automotive Battery:
The primary function of an automotive battery is to store and distribute the electric current to various systems in the vehicle. A modern vehicle needs an automotive battery to operate many electrical components/gadgets. They include electrical gauges, digital devices, power windows, central locking mechanisms, air-conditioning, etc. Nowadays, almost every component operates on an electric current. They range from mobiles and portable refrigerators to electric tire inflators.
Furthermore, the automotive battery not only acts as a storage unit but also as a buffer to the electric components in vehicles. Thus, besides the fuse, it also helps protect the components from a sudden surge of current in case of a fault in the generating system.
Nowadays, many components operate electrically/electronically. They shed their pure mechanical function, which the earlier generation cars used. Today, there are all-electric cars that run only on electric current. Electric cars need to have a better range to travel uninterruptedly. So, the engineers developed an advanced battery version with higher capacity. This battery is known as the Lithium-ion (Li-Ion) battery. It can store more current, which has changed the way cars work.
Types of Automotive Battery according to the technology:
- Lead Acid Battery
- Alkaline Battery
- Zinc Acid Battery
- Lithium-Ion Battery
However, the Lithium-ion battery is the latest and most advanced type. Manufacturers use the Lithium-Ion battery mainly in electric vehicles. This is because it has a higher capacity to store electric energy than other battery types.
Types Of Battery According To The Voltage:
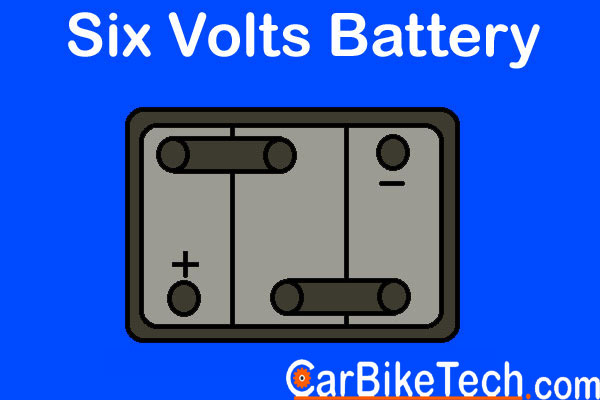
- 6 volt – Typically Used in small bikes
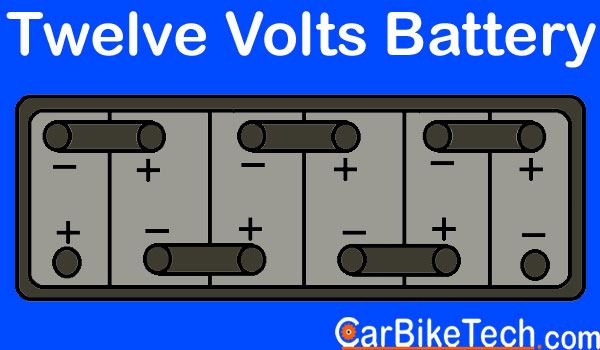
- 12 volt – Typically used in Motorcycles and cars
- 24 volt – Typically used in Commercial vehicles
The Battery’s Main Components:
- Positive Plates
- Separator plates
- Positive terminal
- Negative terminal
- Outer body/Container
Lead-Acid Automotive Battery Construction Details:
Furthermore, the Lead-Acid battery consists of a single-piece molded container. Manufacturers make it of either hard rubber or bituminous material. This material has high acid-proof and insulating properties and greater mechanical strength. Then they divide it into several compartments or cells of the nominal voltage of 2 volts. Usually, there are 6 cells of 2 volts each in a 12 volts battery. The lead bars connect these cells in series. Besides, the battery has two sets of plates or electrodes. Manufacturers dip them in a solution of the diluted sulphuric acid or the electrolyte. One electrode consists of lead dioxide and the other of spongy lead.
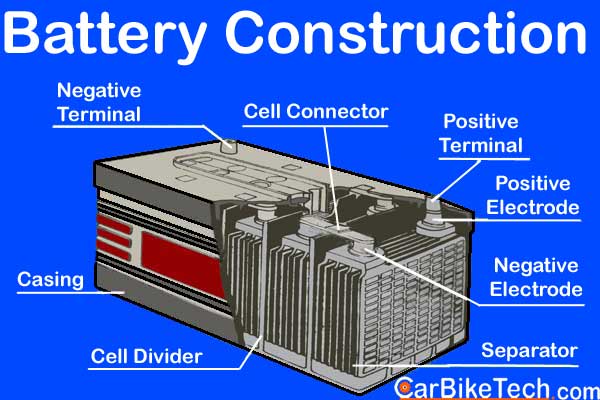
When the cells start to function, the acid reacts with the plates to convert chemical energy into electrical energy. Besides, the negative charge builds up on the lead plate. The positive charge builds up on the lead peroxide plate. The manufacturers use bridges for resting the automotive battery plates at the bottom of each compartment. So, it helps to contain the sediments between the bridge ribs. It also prevents the plates from bridging and shorting by collecting the active material falling from the grid plates into the spaces. The molded cover seals the cell with a removable plug for topping up and testing that individual cell/s. Besides, the manufacturers provide vent holes to allow the escaping of gases released during the charging process.
Following chemical processes that take place during the charging and discharging of a battery:
Discharge
PbO2 + H2SO4 + Pb ↔ PbSO4 + 2 H2O + PbSO4
Charge
(Lead Peroxide + Sulphuric Acid + Spongy Lead) ↔ (Lead Sulphate + Water + Electrolyte)
Bosch, Johnson Controls, Exide, and Lucas are some of the leading battery manufacturers in the world.